You are here
Targeting Stress Granules to Accelerate Nerve Regeneration
Speakers
Abstract
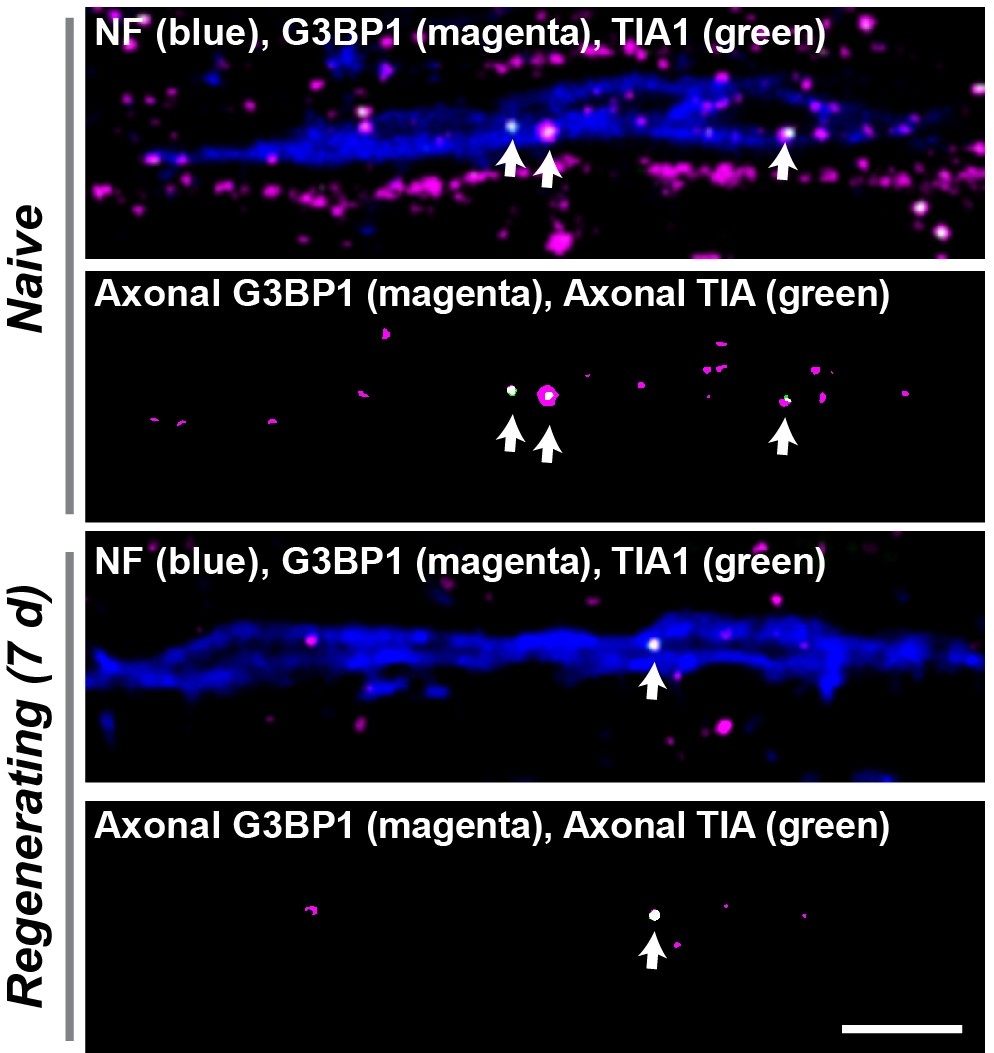
Peripheral nerves spontaneously regenerate after injury but injured spinal cord axons have much lower regeneration capacity. Proteins synthesized locally in axons are needed for peripheral nerve regeneration, and several lines of evidence indicate that injured spinal cord axons have the ability to synthesize proteins when they are made competent to regenerate. We recently published work showing that Stress Granules (SGs) serve as mRNA storage sites in axons through aggregation of the SG protein G3BP1. Axonal SGs attenuate axonal protein synthesis and slow axon growth. We developed viral-based (AAV-G3BP1 B domain) and cell permeable peptide-based (190-208 G3BP1 peptide) strategies to cause axonal SG disassembly, and these approaches accelerate PNS axon regeneration. Our preliminary data show high promise for the SG disassembly approach increasing spinal cord axon regeneration under some settings. Indeed, inhibition of G3BP1 accelerates growth of severed reticulospinal axons in peripheral nerve grafts (PNGs) and shows growth promoting effects in transected CST axons. We also uncovered an endogenous pathway that sensory neurons use for disassembling axonal SGs. Specifically, Casein Kinase 2 (CK2) dependent phosphorylation of axonal G3BP1 triggers disassembly of axonal SGs, releasing axonal mRNAs for translation and increasing axon growth. Temporal and spatial specificity of CK2 in axons is driven by translational regulation of its mRNA (Csnk2a1) in axons after injury.