You are here
The Laboratory for Cortical Interneuron Systems Neuroscience
Our lab works on cortical interneurons, the short-range neurons that provide the sole source of inhibition in the cerebral cortex. Interneurons are highly diverse and act to regulate the flow of cortical information. In the Au lab, we are interested in how interneurons establish circuits in the brain during development and how, if that assembly process goes awry, it can lead to disease. Indeed, there is growing evidence that interneuron dysfunction is linked to numerous diseases, including schizophrenia, autism, Fragile X Syndrome, as well as neurodegenerative disorders such as Alzheimer’s Disease.
With an improved understanding of interneuron dysfunction in disease, we hope to develop more effective therapies for patients. To address this, we use several Experimental Approaches:
To study cortical interneurons, we routinely use Mouse Genetics as a targeted means to
analyze and manipulate interneurons in their native environment. As a complementary approach, we also generate interneurons using Stem Cell Directed Differentiation and Transdifferentiation. This allows us to control the differentiation process and provides a means to study human cortical interneurons. Once generated, stem cell-derived interneurons can be studied in In Vitro Assays for Migration and Synaptogenesis and can also be engrafted into host mouse cortex via Ultrasound Backscatter Microscopy. Recently, we developed a novel approach to analyze cortical interneuron synapses using Computer Vision and Machine Learning. This approach has helped unveil fundamental organizing principles for interneuron synapses and has provided a highly sensitive means to deep phenotype synaptic dysfunction in disease models.
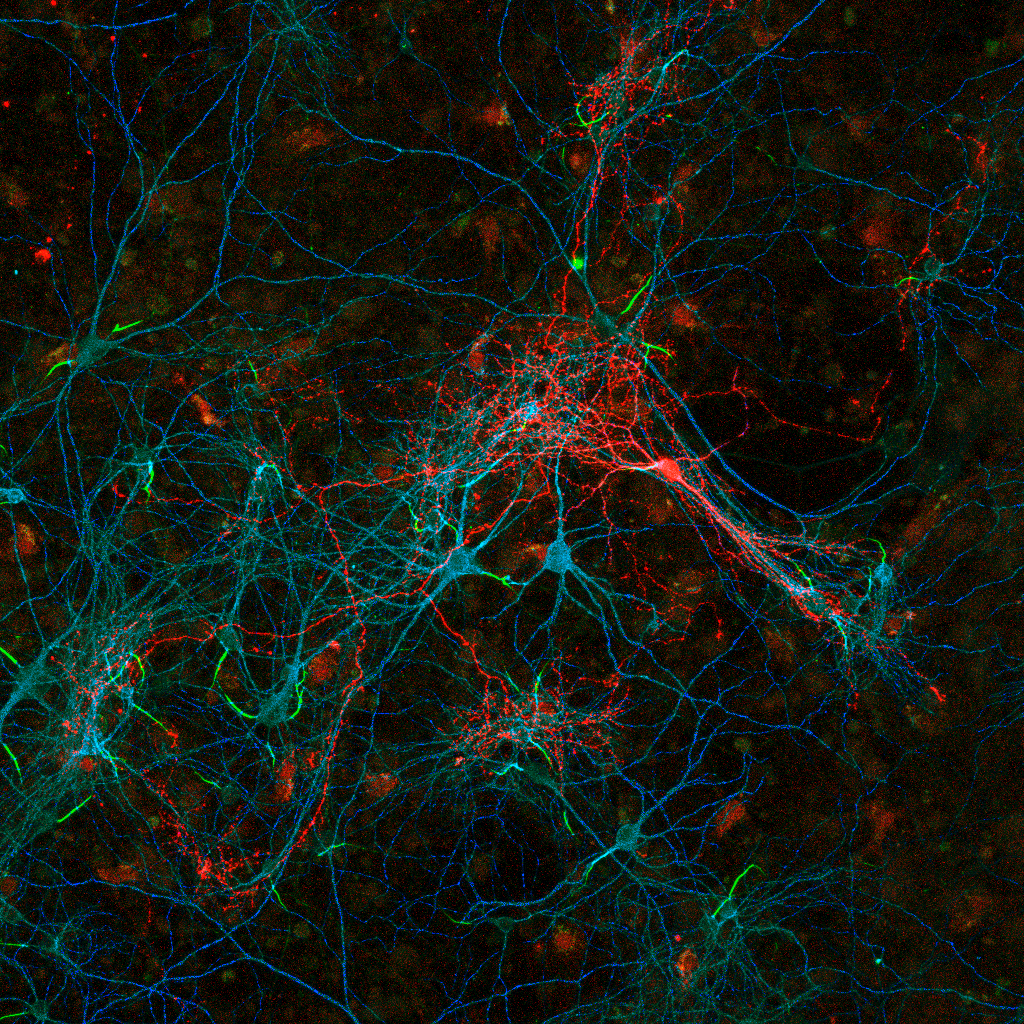
Labeled cortical interneuron (red) in dissociated culture forms synapses onto nearby neuronal cell bodies (cyan), dendrites (blue) and axon initial segments (green) . Individual presynaptic puncta (red) can be analyzed for local spatial and intensity features that provide us with information about synaptic targeting as well as their functional organization onto the postsynaptic neuron.
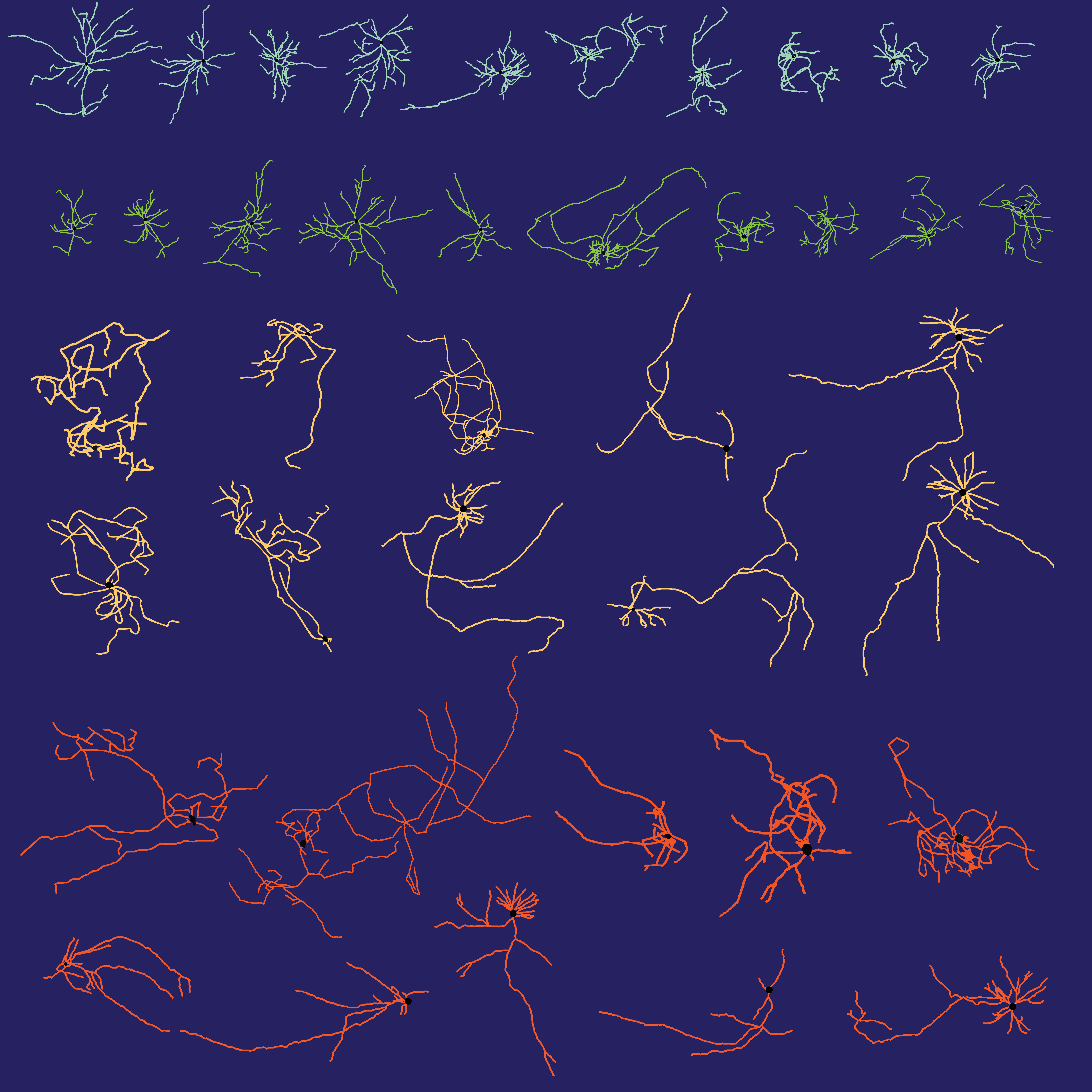
Morphological reconstructions of embryonic stem cell-derived interneurons (top) and projection neurons (bottom). The subpallium produces both populations, and we found that transcriptional specification with St18 (a transcriptional repressor) acts to delineate between the 2 lineages.

A three-panel computer vision-generated depiction of cortical interneuron synapses (magenta) targeting dendrite (left), soma (middle), axon initial segment. We routinely use this approach to extract hundreds of features from each synapse we analyze in order to learn more about how they are functionally organized and how that organization can change in the diseased state.

