You are here
Combined Brain Stimulation and Hand Robotic Training in Chronic SCI
Goal
The purpose of this study is to improve hand function combining transcranial direct current stimulation (tDCS) and innovative interactive hand robotic training in people with chronic SCI. We also aim to investigate the mechanisms of the motor recovery and to characterize the neurophysiological profile of patients and specif i c muscles that respond to robotic motor training by using Transcranial Magnetic Stimulation (TMS).
Techniques
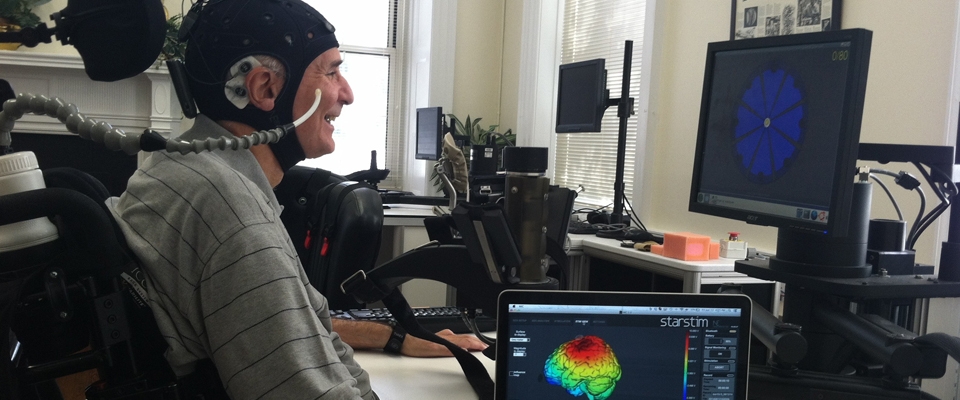
- Transcranial direct current stimulation (tDCS): tDCS is a well-established technique used to induce transient changes in brain excitability. tDCS works by delivering a weak constant direct current through electrodes placed on the scalp of the brain in the area of interest.
- The Hand Robot: The InMotion HAND robot attaches to the InMotion ARM robots and provides assist-as-needed gross grasp and release motion. It also works together with the InMotion ARM robot to provide assist-as-needed support for functional reach-grasp-release movement. There are several distinct protocols of assessment for the InMotion HANDTM robot. The Grasp Adaptive has the patient grasp and release provides patient performance metrics over 80 movements. The Reach Adaptive requires the patient to reach, grasp and release with both InMotion ARMTM and InMotion HANDTM. In this scenario, the performance metrics are only provided for the “reach” portion of the activity. The Pick Adaptive requires a patient to reach, grasp, hold, and release a target. In sum, the InMotion HANDTM robot will provide us a fine evaluation of kinematic aspects of hand function in a SCI population.
- Transcranial Magnetic Stimulation (TMS): TMS is a useful and safe tool that sends brief currents via an insulated coil to painlessly generate currents in the brain tissue beneath the coil. TMS will be placed over the subject’s skull over the area of the brain that controls hand movements to assess the brainmuscle connections.
Study Protocol
The combined tDCS and hand robotic training is a 6-week program that includes 3 sessions per week, each session lasting approximately 1 hour (18 sessions total). The hand robotic training will be preceded by 20 min anodal 2mA tDCS or sham stimulation. The participants will be evaluated before, after and in a 1 month follow-up. Participants will be assessed on clinical/functional evaluation, TMS and robotic kinematics.
Eligibility
- Age: 18 to 75 years old.
- Injury Date: Greater than 6months af ter injury.
- Injury Type: Cervical lesion. Traumatic/Non-traumatic. ASIA B, C, D.
- Presence of some degree of weakness in the hand.
- Ability to pick at least one block in the Box and Blocks test.
- Ability to tolerate sitting upright at for at least one hour.
- Cognitively and behaviorally capable of complying with the regimen.
- Concomitant/progressive neurological disorder.
- ASIA A (motor and sensory complete).
- Medically unstable.
- Severely limited range of joint motion or irreversible muscle contractures in the upper limb.
- Less than 6 months post-injury.
- Level of injury caudal to T1 level.
- Presence of contraindications for TMS/tDCS:
- Implanted metal devices in the head (as an intra-cerebral vascular clip or any other electrically sensitive support system).
- Presence of cardiac pacemaker.
- Past Medical history of seizures or unexplained spells of loss of consciousness.
- A history of medication-resistant epilepsy in the family members.



