You are here
Redox Regulation, Protein S-Nitrosylation, and Synapse Loss in Alzheimer’s Disease and Related Dementias
Speakers
Abstract
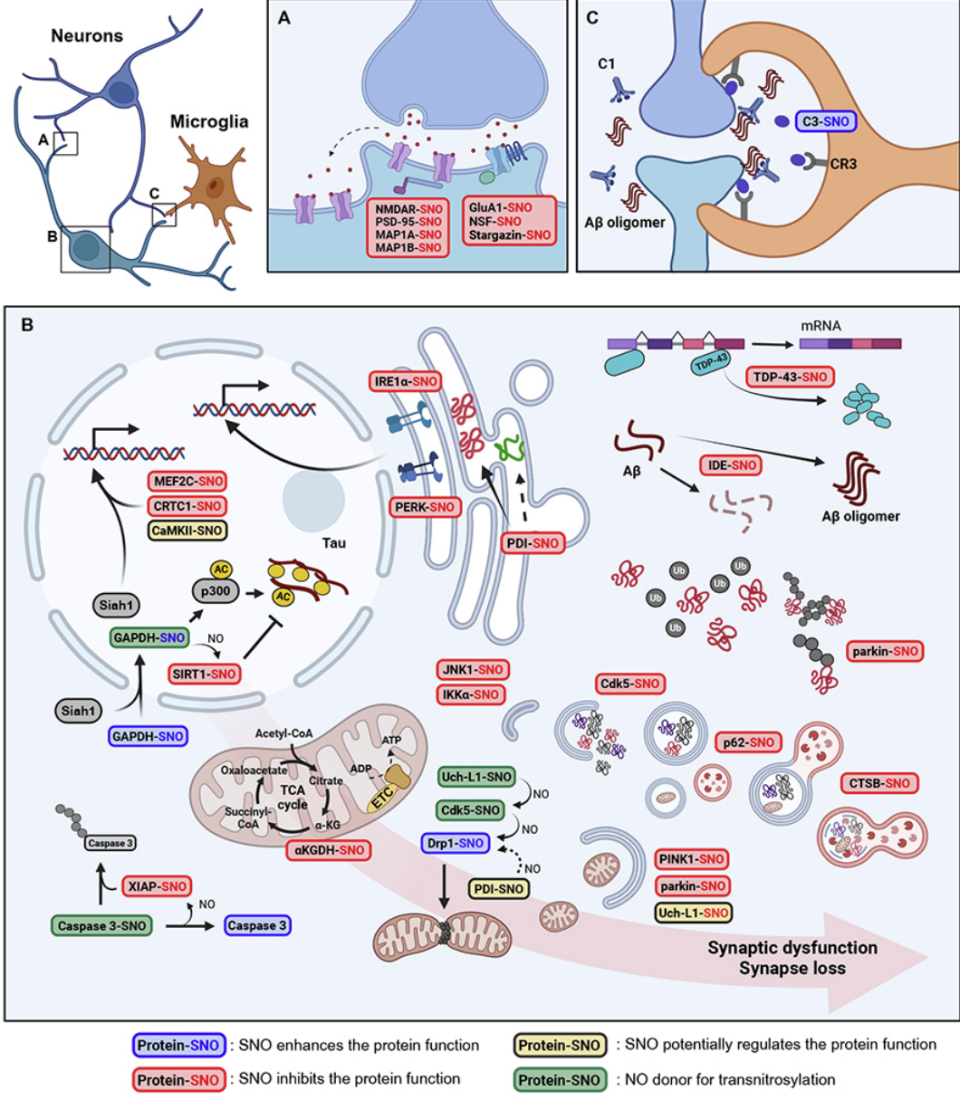
Redox regulation, protein S-nitrosylation, and synapse loss in Alzheimer’s disease and related dementias Redox-mediated posttranslational modification, as exemplified by protein Snitrosylation, modulates protein activity and function in both health and disease. Here, we review recent findings that show how normal aging, infection/ inflammation, trauma, environmental toxins, and diseases associated with protein aggregation can each trigger excessive nitrosative stress, resulting in aberrant protein S-nitrosylation and hence dysfunctional protein networks. These redox reactions contribute to the etiology of multiple neurodegenerative disorders as well as systemic diseases. In the CNS, aberrant S-nitrosylation reactions on single proteins or, in many cases, interconnected networks of proteins lead to dysfunctional pathways affecting ER stress, inflammatory signaling, autophagy/mitophagy, the ubiquitin-proteasome system, transcriptional and enzymatic machinery, and mitochondrial metabolism. Aberrant protein S-nitrosylation and transnitrosylation (transfer of nitric oxide (NO)-related species from one protein to another) trigger protein aggregation, neuronal bioenergetic compromise, and microglial phagocytosis, all of which contribute to the synapse loss that underlies cognitive decline in Alzheimer’s disease and related dementias as well as neurodevelopmental maladies such as autism spectrum disorder.

