You are here
Cerebroprotection for Acute Ischemic Stroke: Looking Ahead
Speakers
Abstract
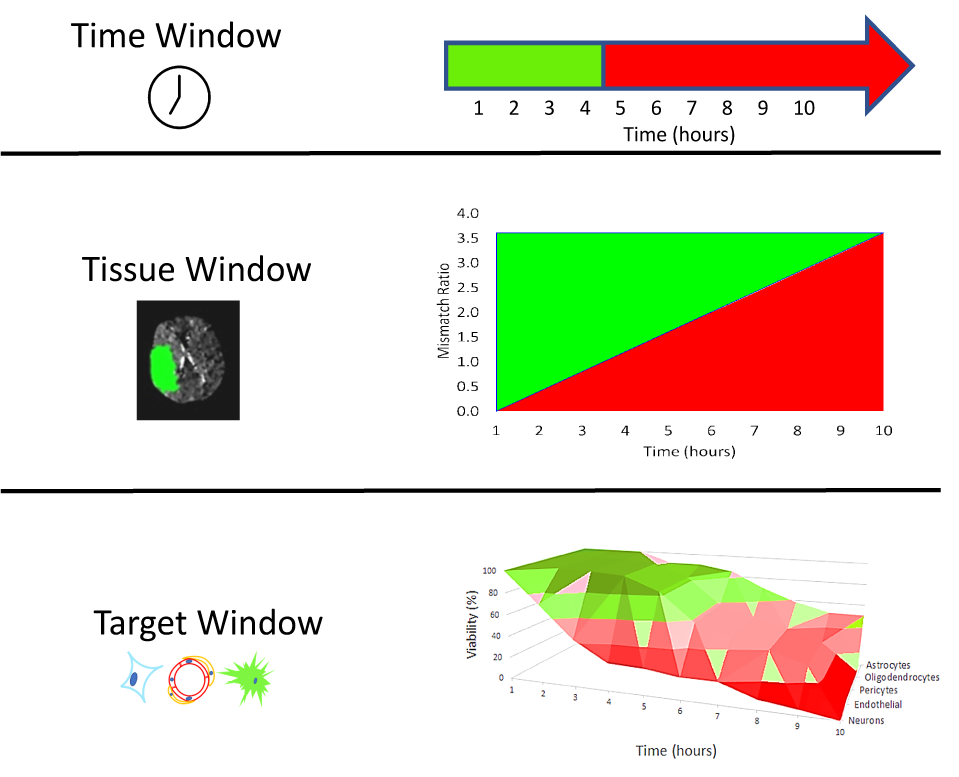
Despite years of basic research and pioneering clinical work, ischemic stroke remains a major public health concern. Lessons can be derived from previous failures that may point to new directions and new strategies. In previous development efforts, the basic science and preclinical assessment of candidate treatments often lacked rigor and sufficiency; the clinical trials may have lacked power, rigor, or rectitude; or most likely both preclinical and clinical investigations were flawed. Single-target agents directed against specific molecular mechanisms proved unsuccessful. Elements of the neurovascular unit show differential vulnerability evolving over differing time scales in different brain regions. To move forward, the term neuroprotection should be replaced as it has become ambiguous: protection of the entire neurovascular unit may be called cerebral cytoprotection or cerebroprotection. Success in developing cerebroprotection—either as an adjunct to recanalization or as stand-alone treatment—will require new approaches that recognize the importance of differential vulnerability in the neurovascular unit. Recent focus on pleiotropic multi-target agents that act via multiple mechanisms of action to interrupt ischemia at multiple steps may be more fruitful. The term “brain cytoprotection” or “cerebroprotection” to replace the term ‘neuroprotection’ when the intention of an investigation is to demonstrate that a new, candidate treatment benefits the entire brain. Although “time is still brain,” tissue imaging techniques have been developed to identify patients with both predicted core injury and penumbral, salvageable brain tissue, regardless of time after stroke symptom onset. Based on contemporary principles of rigor and transparency, revised and enhanced the preclinical recommendations for developing new treatments are proposed to proceed in two phases: an exploratory ‘qualification phase’ and a definitive ‘validation phase’. Prior to clinical trials, testing of candidate molecules in stroke models could proceed in a comprehensive manner using animals of both sexes and to include significant variables such as age and comorbid conditions. Comprehensive preclinical assessment might include multi-center, collaborative testing, e.g., network trials. All of these changes are recommended to end the procession of repeated translational-clinical failures.