You are here
Stem Cell Flexibility Underlying Brain Development and Repair
Speakers
Abstract
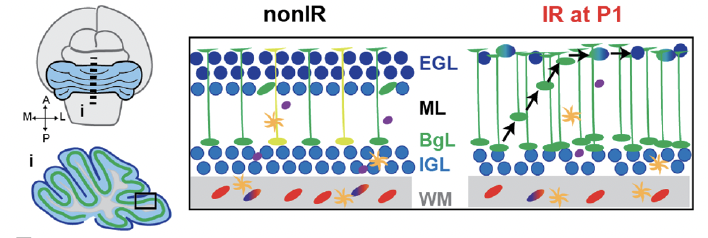
The mouse neonatal cerebellum has a remarkable capacity to regenerate neurons killed around birth. Mouse and human cerebela have distinct stem cell populations in the first weeks and months, respectively, after birth: 1) ventricular zone-derived Nestin-expressing progenitor (NEP) populations that produce interneurons and astroglia and 2) rhombic lipderived granule cell progenitors (GCPs) that generate excitatory neurons. We demonstrated that when mouse Purkinje cells are ablated at P1, they are efficiently replaced by a rare population of immature Purkinje cells capable of proliferation and differentiation (Bayin, 2018). In a second series of studies, we uncovered that a subset of astroglia-committed NEPs change their fate to become excitatory granule cells in response to injury to the GCPs at P1 (Wojcinski, 2017; Wojcinski, 2019; Yang & Joyner, 2019). Using genetic labeling, fate mapping and live imaging we found that NEPs in the Purkinje cell layer rapidly respond to the injury by expanding, migrating to the GCP niche and adopting a granule cell molecular profile. White matter NEPs instead delay their production of interneurons and astrocytes, thus maintaining circuit cell proportions, and rescuing motor behaviors. Furthermore, single cell sequencing (scRNAseq) analysis and conditional mutagenesis uncovered that upregulation of the neurogenic transcription factor ASCl1 in gliogenic-NEPs is necessary for their glial-to-neural fate change (Bayin, 2021). Additional studies include determining whether NEPs in the adult cerebellum can be stimulated to replenish cells lost due to injury and testing the requirement of ROS signaling in driving adaptive reprogramming.