You are here
Optogenetic Modulation of TDP-43 Proteinopathy
Speakers
Abstract:
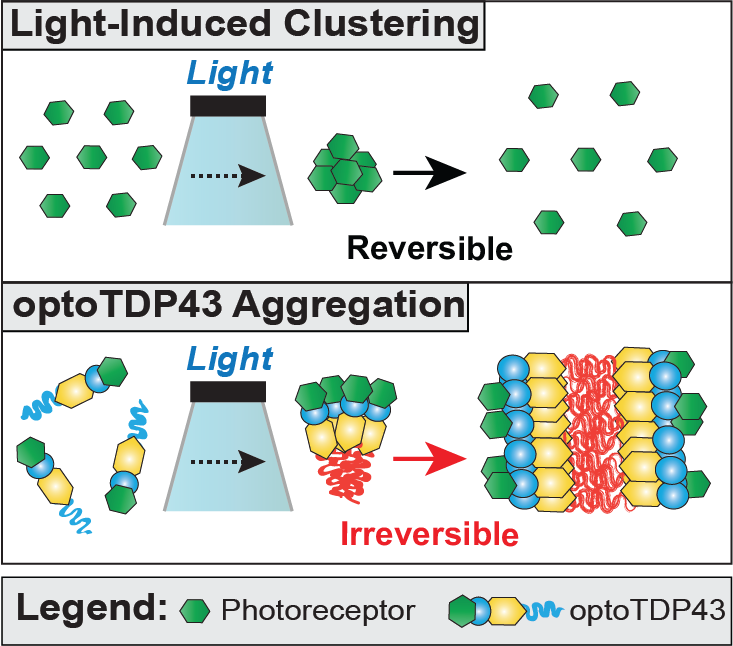
Pathological hallmarks of age-related neurodegenerative diseases are intracellular protein inclusions. TDP-43 is a predominantly nuclear DNA/RNA binding protein but is found mislocalized to the cytoplasm and aggregated in a variety of neurodegenerative diseases. TDP-43 proteinopathy is a neuropathological feature of amyotrophic lateral sclerosis, a fatal disease characterized by the degeneration of the motor neurons of the brain and spinal cord, and a predominant pathology in frontotemporal dementia, the most common cause of dementia in individual under the age of 65. Modeling TDP-43 and other neurodegenerative proteinopathies is challenging and relies on nonphysiological overexpression of wild-type or mutant variant of the proteins. This approach lacks any control over the aggregation process. Furthermore, these models do not often mimic key features of the diseases in patients. To overcome this problem, we developed a photokinetic technique that allows for the induction of neurodegenerative protein oligomerization under spatial and temporal control. Employing this light-based/optogenetic approach, we identified the intracellular requirements for the development of TDP-43 proteinopathy and identified mechanisms to disrupt the formation of these pathological inclusions.