You are here
Neurovascular Protection by Adropin in Experimental Ischemic Stroke
Speakers
Abstract
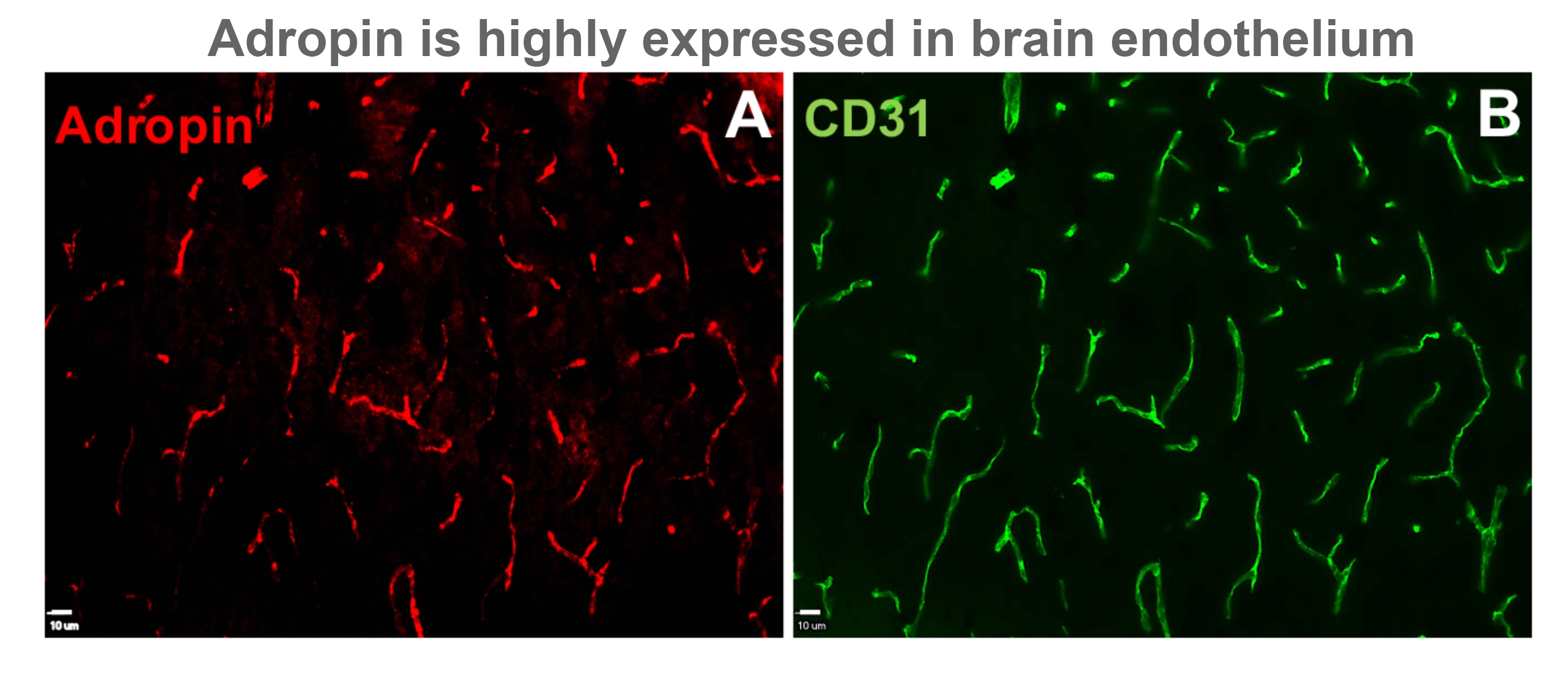
Adropin has been shown to improve peripheral endothelial dysfunction, reduce insulin resistance, and increase glucose utilization in obesity and diabetes. We recently showed that adropin reduces paracellular permeability of brain endothelial cells exposed to ischemia-like conditions in vitro. Here we hypothesized that adropin exerts neuroprotection through activation of endothelial nitric oxide synthase (eNOS)/NO signaling pathway and reduction of blood-brain barrier (BBB) damage. Male mice were subjected to permanent middle cerebral artery occlusion (MCAO) and treated intravenously with either vehicle or synthetic adropin (90, 900 and 2700 nmol/kg) at the onset of ischemia and sacrificed to determine infarct size at 48 h. Brain tissue was collected at 24 h after stroke to determine BBB damage, matrix metalloproteinase (MMP)-9 activity, and levels of tight junction proteins, eNOS, and gp91phox-containing NADPH oxidase. We determined the effects of a 3-h delayed administration on stroke outcomes to evaluate the translational value of adropin therapy. Adropin given at the onset of MCAO dose-dependently reduced infarct size. More importantly, delaying adropin administration to 3 h post-MCAO profoundly reduced infarct size compared to the vehicle group. Ischemia slightly increased eNOS phosphorylation at Ser1176, significantly increased gp91phox (a major source of reactive oxygen species), and reduced endogenous brain adropin levels. Adropin treatment induced a dramatic increase in eNOS phosphorylation, significantly reduced gp91phox levels, and restored endogenous adropin levels compared to the vehicle group. Additionally, adropin protected against a stroke-induced increase in MMP-9 activity, loss of zona occludens-1 and occludin, and BBB disruption as assessed by extravasated plasma proteins (IgG, albumin, and hemoglobin). Adropin protection was completely abolished in eNOS deficient mice suggesting an eNOS-dependent mechanism underlying the protective effects of adropin in stroke. We also showed that transgenic overexpression of adropin significantly reduced infarct size and BBB damage, while genetic deletion (adropin knockouts) dramatically increased brain injury in vivo following stroke. Collectively, these findings indicate that adropin is a novel mediator of neurovascular protection following ischemic stroke.