You are here
Neuroimmune Interactions in Chronic Pain: From Clinical to Clinically-Informed Basic Science
Speakers
Abstract
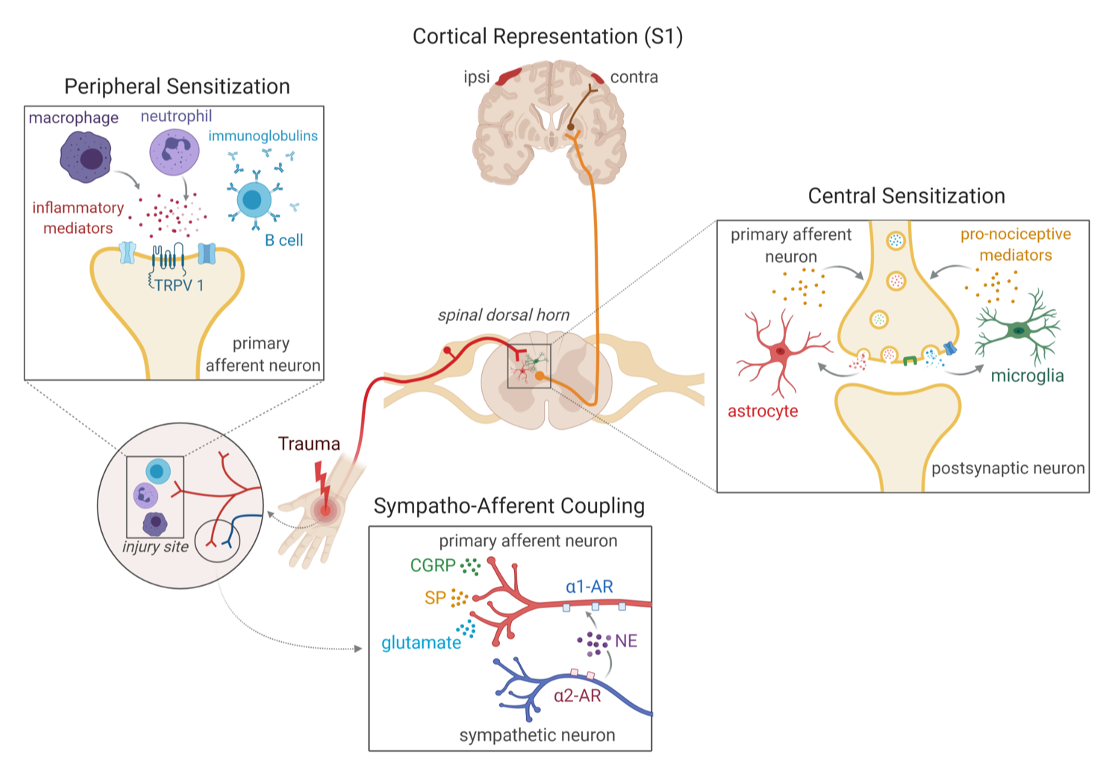
The mission of the Tawfik Lab is to do the best clinically-informed basic science research to advance our understanding of the neuroimmune contribution to chronic pain in a thoughtful manner with our patients always in mind. We are particularly interested in understanding the unique underpinnings of various types of chronic pain and how central nervous system (CNS) glial cells (astrocytes and microglia) contribute to the transition from acute to chronic pain. Microglia are particularly interesting to us as the macrophages of the CNS with known roles in synaptic pruning and neuroinflammation.
Activated myeloid-lineage cells, macrophages peripherally and microglia centrally, contribute to the acute-to-chronic pain transition, however, the details on the timing and possible sex-specificity of such involvement remains a matter of debate. For example, there is evidence that CNS microglia may contribute to chronic pain only in males. In this talk I will discuss data from my laboratory using complementary pharmacologic and transgenic approaches in mice to more specifically manipulate myeloid-lineage cells using a model of the pain condition, complex regional pain syndrome. I will discuss a novel spatiotemporal transgenic mouse line, Cx3CR1-CreERT2-eYFP;TLR4fl/fl (TLR4 cKO) that we used to specifically knock out toll-like receptor 4 (TLR4), only in microglia and no other myeloid-lineage cells. Using this transgenic mouse, we find that early TLR4 cKO results in profound improvement in chronic, but not acute, allodynia in males, with a significant but less robust effect in females. In contrast, late TLR4 cKO results in partial improvement in allodynia in both sexes, suggesting that downstream cellular or molecular TLR4-independent events may have already been triggered. I will further discuss new data using a transgenic mouse that allows for microglia-specific depletion, Cx3CR1-CreERT2-eYFP;iDTRlox-STOP-lox (microglia cKO). We performed microglial depletion at multiple time points after peripheral injury and see the most striking decrease in mechanical allodynia in males and females when depletion is performed several weeks after injury. Overall, we find that microglia themselves contribute to the chronic pain transition in both sexes, however, microglial TLR4 contributes more heavily to the transition in males.