You are here
Ion Mobility Mass Spectrometry Reveals Hidden Post Translational Modifications of Amyloid Beta in Alzheimer’s Disease Brain
Speakers
Abstract
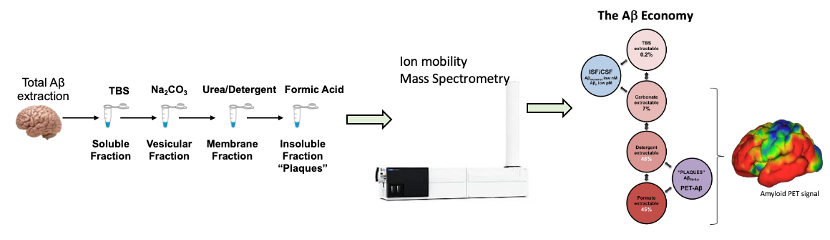
Part of the quest to understand neurodegenerative research requires a detailed accounting and measurement of the biological components in neurological cells, tissue and biofluids. Regulation of the abundance of proteins is one mechanism of regulating function and post-translational modification (PTM) is another. PTMs are often invisible to targeted assays like ELISA or westerns this includes modifications including phosphorylation and, more recently, the hidden isomeric modifications (e.g. iso-aspartate). Here we apply quantitative proteomics and ion mobility mass spectrometry to investigate the isomerization of amyloid beta peptide from human brain tissue. To our surprise we discovered that isomerization at aspartic acid makes up over 80% of the total peptide in Alzheimer’s disease brain. We show that the implementation of quantitative mass spectrometry and ion mobility reveal hidden biology and improve our understanding of the role of amyloid beta peptide in Alzheimer’s disease. We will also discuss the role of protein biomarkers for the diagnosis of AD.