You are here
Mechanisms of Pain Following Severe Spinal Cord Injury
Speakers
Abstract
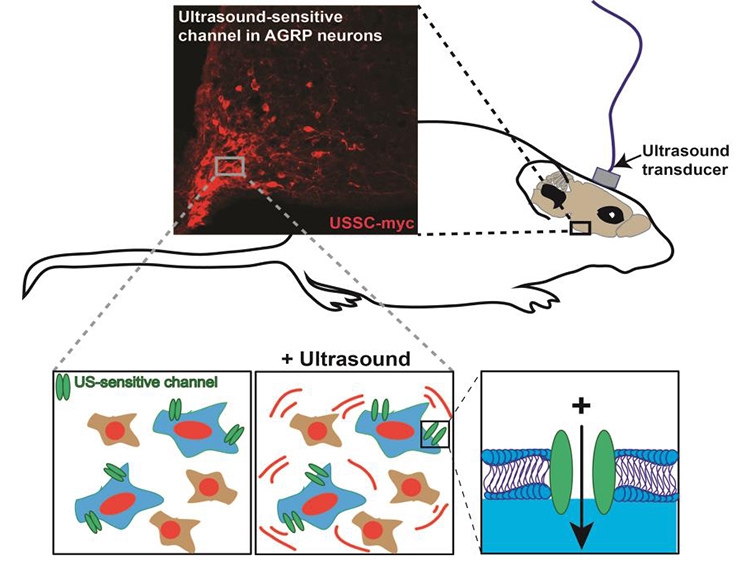
Neuropathic pain is a severe and debilitating consequence of spinal cord injury (SCI) that occurs in up to 53% of the SCI population. However, current treatments are frequently ineffective, in part because the etiology of SCI pain is poorly understood. SCI pain research has primarily focused on below-level hindlimb responses to tactile and heat stimuli, which bear little resemblance to the clinical manifestation of below level pain. Therefore, we characterized a model of severe T3 SCI and the resulting at-level pain that develops in the forelimbs, focusing on pain modalities that mimic the clinical description of at-level pain and including an emotional-affective dimension. We further found that at-level SCI pain was associated with upregulation of spinal and dorsal root ganglia GFAP expression, indicative of reactive astrogliosis and reactive satellite cells, respectively. This was accompanied by increased expression of the gap-junction protein, connexin-43, which allows direct communication between adjacent astrocytes or satellite cells. Pharmacological blockade of connexin-43 gap junctions alleviated neuropathic pain behavior in SCI rats, implicating connectivity between adjacent astrocytes of satellite cells in the pathogenesis of SCI neuropathic pain. However, given the many different common symptoms of neuropathic SCI pain, including spontaneous pain and hypersensitivity to touch and cold, identification of the circuits and neuronal subtypes that give rise to these different pain modalities may be critical to informing therapeutic development. To facilitate this type of research, we are currently developing “sonogenetics”- a tool that allows non-invasive activation of neurons using ultrasound.