You are here
Circuit Repair Laboratory
The Hollis lab has four main areas of focus. We are interested in: 1) the general response of neural architecture to spinal cord injury; 2) the function of neuronal circuits underlying movement; 3) the recovery of neuronal circuits after injury; 4) molecular control over glial responses to CNS injury. Our studies use genetic, molecular, and behavioral tools along with optogenetic stimulation of, and optical recording from, neuronal networks in order to understand mechanisms of spinal cord injury and neural circuit remodeling.
Spinal cord injury results in the disruption of neuronal circuits in the spinal cord, neuronal and glial cell death, inflammation, secondary degeneration, scarring and the disconnection of local circuits from supraspinal networks. After injury, a limited amount of endogenous recovery is possible, likely driven by the plasticity of neural circuits in close proximity to the lesion site or from compensatory function from supraspinal projections. The cortical mechanisms supporting this recovery are not well understood, nor is it known whether limitations on plasticity of cortical networks underlie the failure of current therapies to restore lost function.
Our goal is to develop novel therapeutic interventions that take advantage of circuit plasticity to promote recovery from neurological injury. To do so, we are investigating all levels of the circuits supporting movement. Within the spinal cord, we are studying developmental regulation of axon regeneration, as well as mechanisms of the astrocyte response to injury, in order to limit secondary degeneration and maintain local circuit survival. In the periphery, we are targeting pro-regenerative pathways to improve surgical interventions that support the return of hand function in individuals living with chronic cervical spinal cord injury. Within the cortex, we are focused on recovery and plasticity of the sensory and motor networks required for interpreting and regulating skilled movement and motor learning.
Ongoing projects in the lab include:
- Determining the role of epithelial-to-mesenchymal transition mechanisms in the astroglial response to spinal cord injury.
- Measuring the ability of cortical motor networks to incorporate regenerated axons after injury.
- Determining the role of neuromodulation of motor circuits in coordinated motor learning and recovery after spinal cord injury.
- Measuring the changes in intracortical architecture that occur in response to rehabilitation from spinal cord injury.
- Testing the extent of circuit remodeling driven by nerve transfer surgery to treat chronic, cervical spinal cord injury (clinical and pre-clinical studies).
- Exploring the conserved cellular and molecular mechanisms underlying peripheral regeneration.
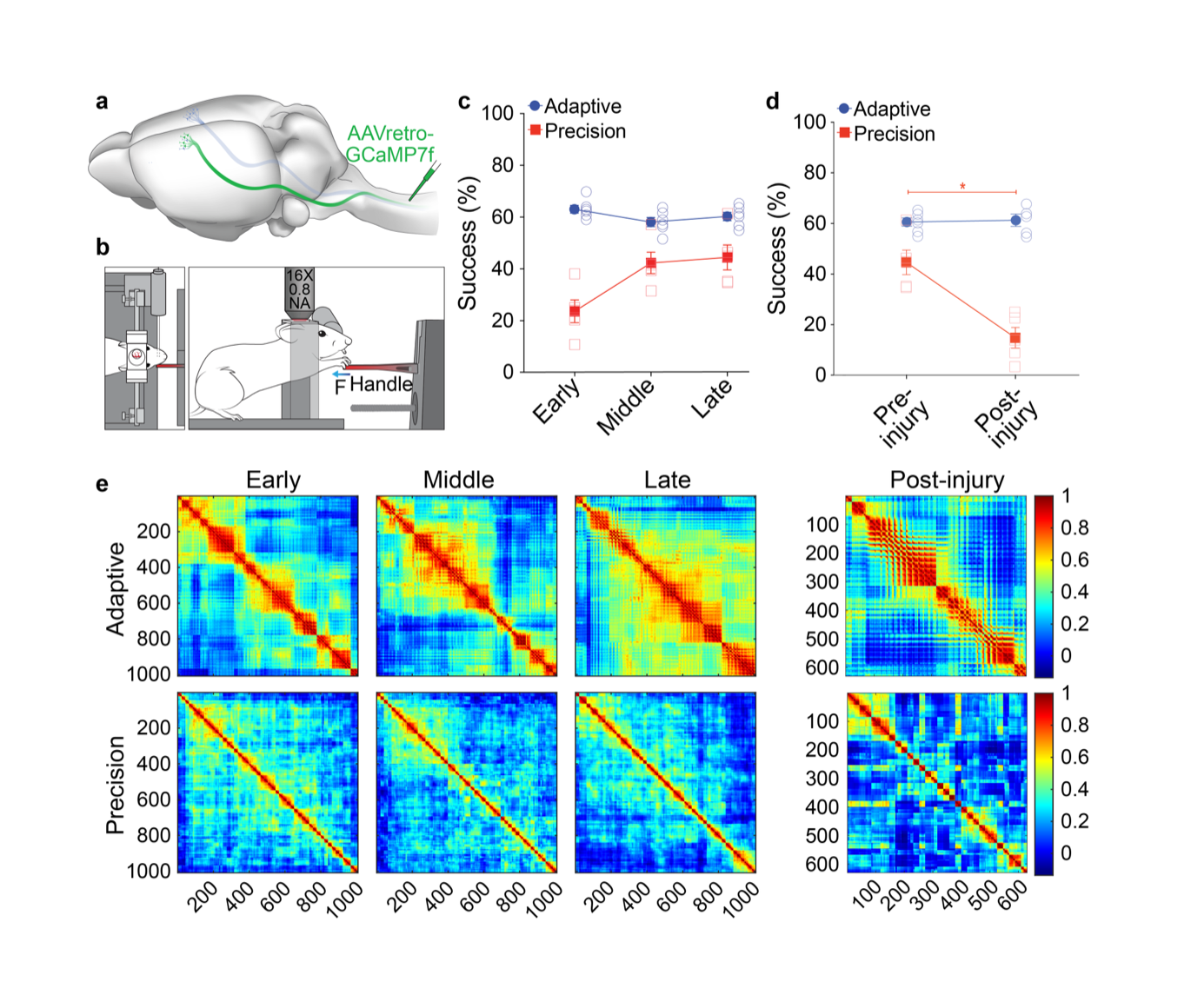
Figure 1: Learning of precision motor control shapes corticospinal motor networks. a) Illustration of retrograde transduction of corticospinal neurons. b) Illustration of head-fixed behavior during imaging. c) Success rate on isometric pull tasks at three distinct training phases. Mice were proficient on a simple, adaptive isometric pull early in training, whereas mice had to learn our skilled, precision isometric pull task. d) Successful task execution is impaired on precision, but not adaptive, isometric pull following bilateral pyramidotomy. e) Correlation coefficients from principal components analysis plotted in heatmaps where each point is the coefficient from a neuron pair. There is more widespread network activation in adaptive pull with pairs of neurons showing higher correlation in activity during movements. Precision coefficients are smaller indicating diverse activity of individual neurons during movement.
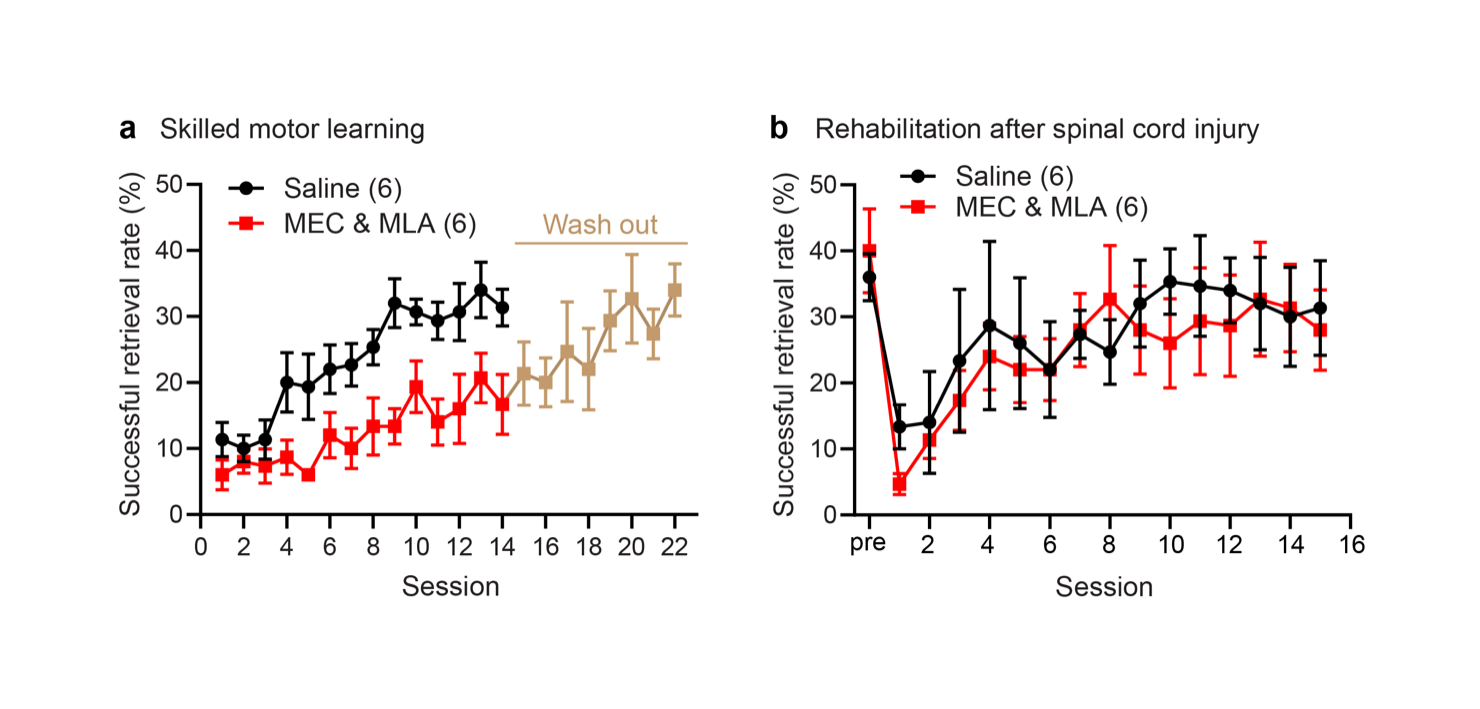
Figure 2: Nicotinic acetylcholine signaling is required for motor skill learning but not for recovery of the same skill after spinal cord injury. a) Nicotinic acetylcholine inhibitors mecamylamine (MEC) and methyllycaconitine (MLA) significantly attenuated learning of the recessed single pellet reach task. Five days after MEC and MLA treatment was stopped, mice trained for an additional 8 days were able to learn the task. b) Functional recovery through rehabilitation after cervical level 5 spinal cord injury. Animals injected with MEC and MLA show similar functional recovery on the single pellet reach behavior to vehicle-treated control animals.
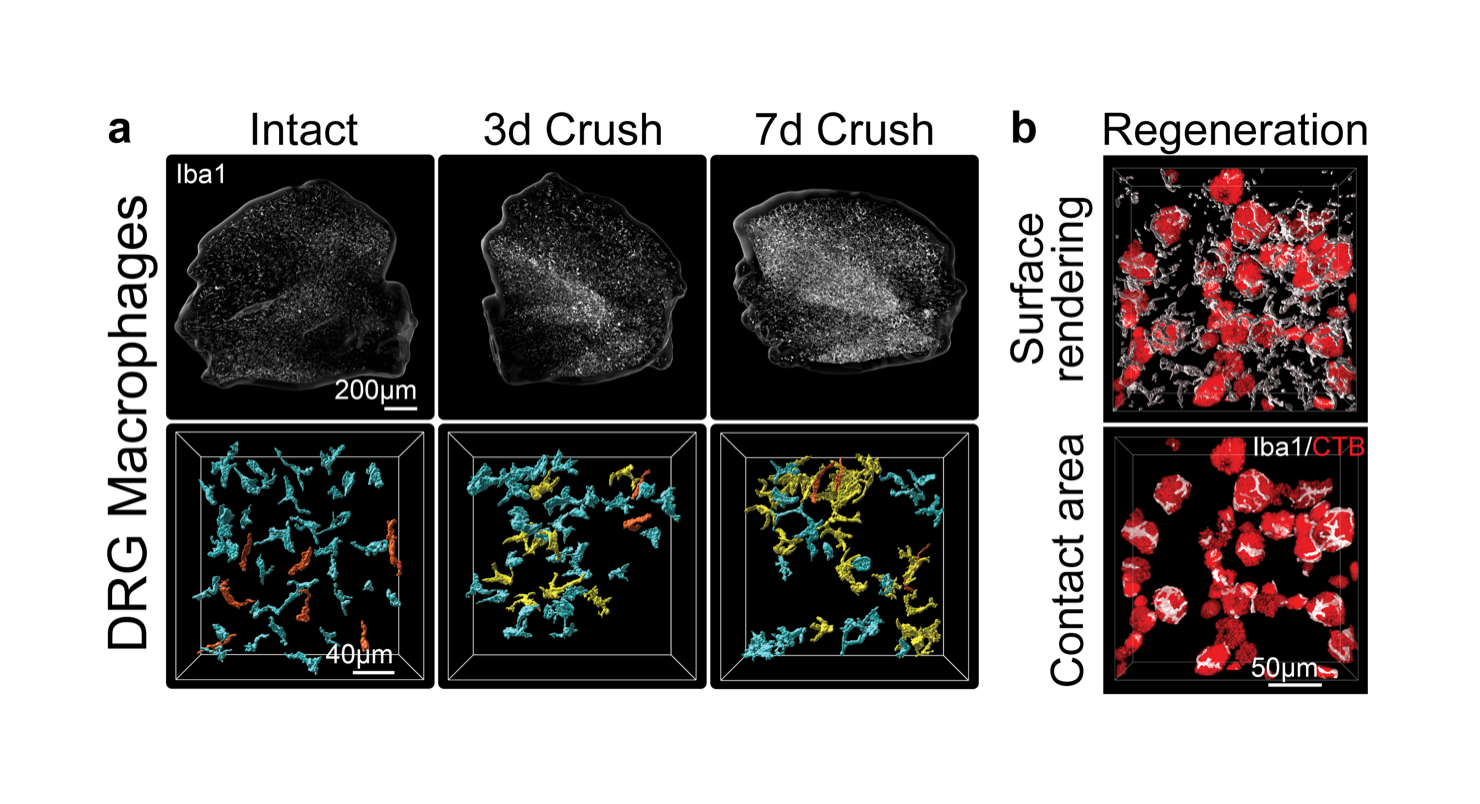
Figure 3: Sciatic nerve crush triggers macrophage interactions with regenerating sensory neurons in the dorsal root ganglia (DRG). a) Macrophages labeled with Iba1 antibody in iDisco cleared L4 DRGs from intact, 3, and 7 days post-injury. b) IMARIS 3D reconstructions show macrophage contacts with regenerating sensory neurons labeled with retrograde tracer CTB at 7 days after nerve crush.

