You are here
Stroke Neuroimmunology Laboratory
Although stroke is a primary cause of disability and death in the U.S., few options exist for treating patients who have had a stroke. The paucity of treatment options has prompted the field to focus on identification and validation of potential targets for developing treatment strategies. There have been numerous translational failures based on acute neuroprotective strategies in preclinical stroke studies. In addressing the translational gap, our research program has investigated comorbidity-modified stroke pathology in animals including hyperlipidemic, diabetic and obese conditions. Inclusion of these comorbidities in our experimental model of stroke provided understanding in disparities of stroke pathology between normal and metabolically compromised conditions. We have defined immune mechanisms involving neural inflammation and tissue repair processes using immunologic, molecular and system approaches and have proposed intervention strategies tailored to specific comorbid conditions.
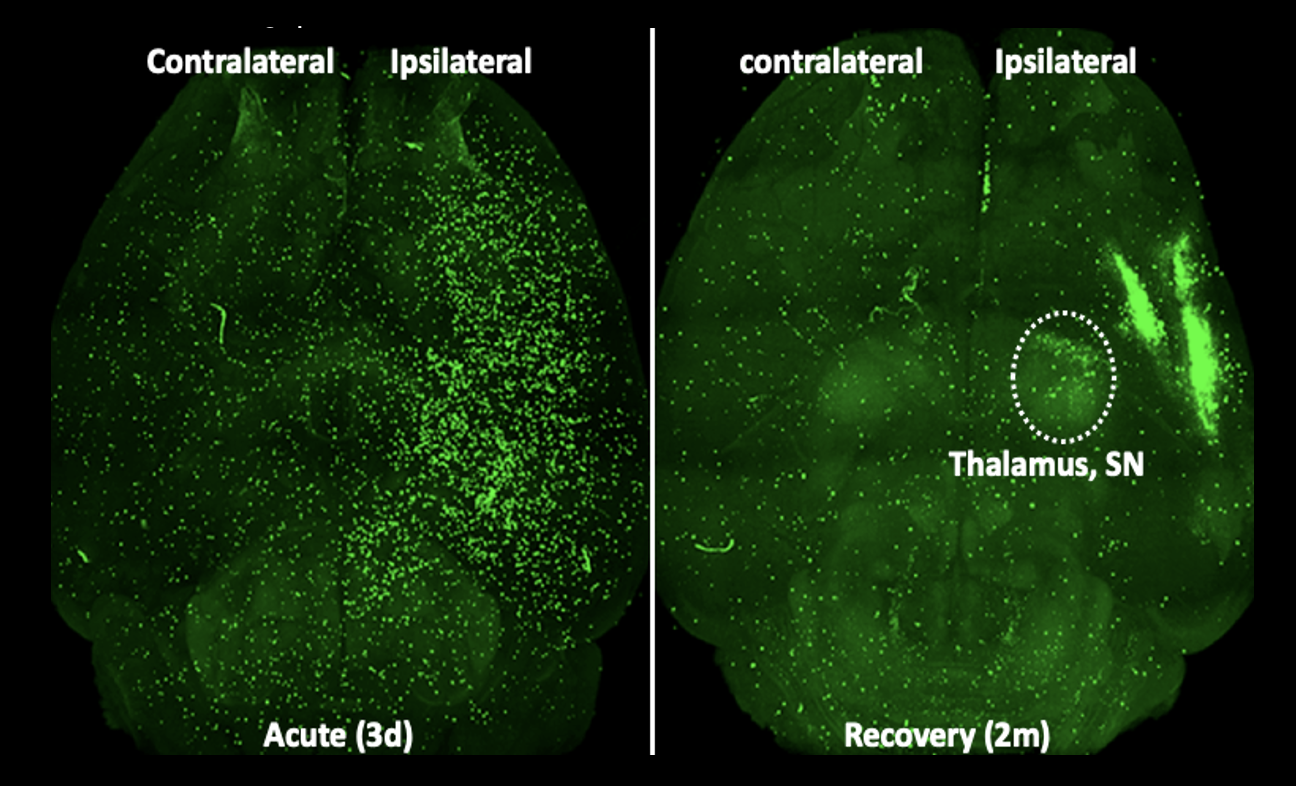
Figure 1. Stroke-induced peripheral immune cell trafficking to the brain at acute (3d) and recovery (2m) stage of stroke. Green fluorescence protein (GFP+) splenocytes was adoptively transferred to asplenic (removal of spleen) mice one day prior to sacrifice. Representative horizontal view images from cleared whole brain at 3d and 2m post-stroke.
Despite stroke being historically considered a CNS event, peripheral immunity is altered by cerebral ischemia and influences the progression of brain injury in stroke. By establishing a long-term stroke recovery model in an experimental animal model of stroke, we have addresses pathology and recovery/remodeling processes in a comprehensive context of stroke, with specific focus on understanding of neuro-immune interactions between CNS and peripheral system from acute to recovery phases of stroke. The formulated immune-based strategies aim at promote functional recovery in chronic stroke facilitates our translation-focused preclinical program for future clinical validation and application.
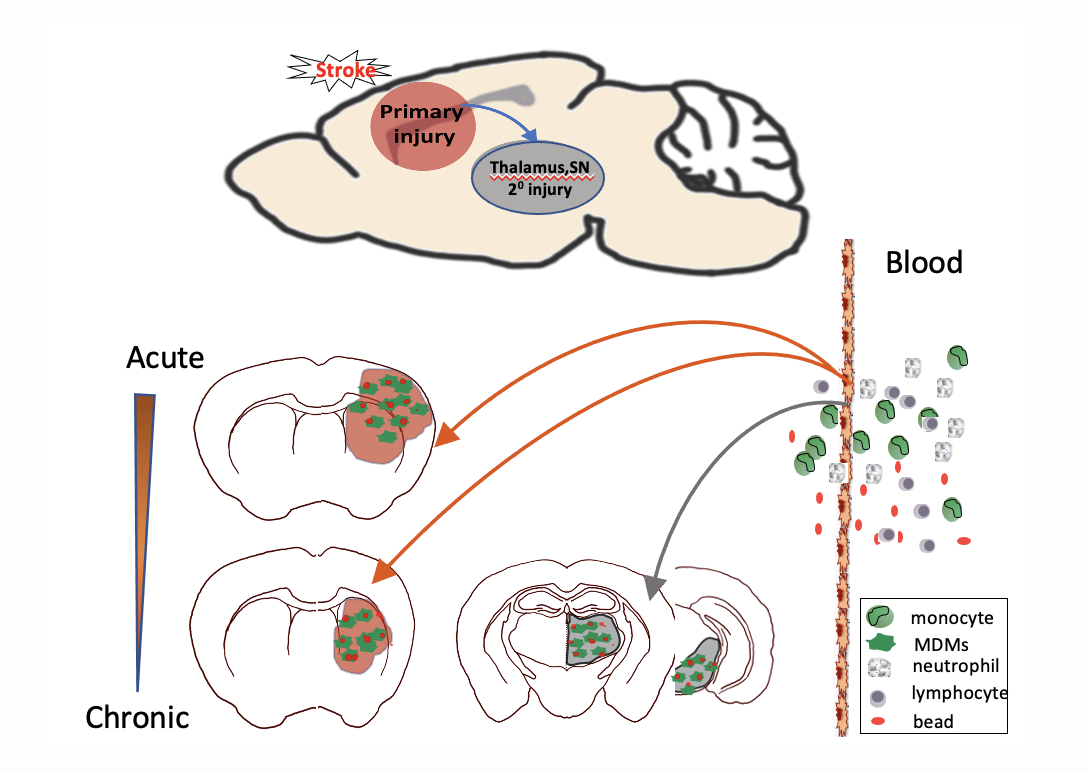
Figure 2. Spatiotemporally regulated monocyte trafficking and brain atrophy in chronic stroke. Circulating monocytes infiltrate to the injured brain in a stepwise manner with an acute and prolonged infiltrations into the primary injury sites involving the striatum and cortex, followed by delayed entry to remote areas such as thalamus and substantia nigra (SN). Monocyte-derived macrophages (MDMs) display high phagocytic activity and contribute to progressive secondary (20) degeneration in the thalamus and SN.

