You are here
Reversible Redox Regulation of Cytoskeletal Dynamics to Control Neuronal Form and Function
Speakers
Our brains control such diverse abilities as movement, sensation, intelligence, speech, emotion, and memory only
because our neurons communicate with one another and the rest of our body through highly organized networks.
These neuronal networks or connections are formed when neurons send out their stalk-like axon and dendrite
appendages. To accomplish this, neurons require actin and tubulin proteins to assemble together into long
polymers (F-actin and microtubules, respectively) – and numerous extracellular stimuli have now been identified
that alter the assembly and organization of these cytoskeletal structures. Yet, we still know little of how these
extracellular cues exert their precise effects on the cytoskeleton. To better understand these mechanisms, my
lab has been focusing on one of the largest families of extracellular cues, the Semaphorins (Semas) – which alter
neuronal behaviors by eliciting destabilizing effects on both F-actin and microtubules. Our strategy has been to use
model organisms and screening approaches to search for proteins that work in the signal transduction cascade
utilized by Semas and their Plexin receptors. Among the proteins that we have identified, is a new family of
intracellular proteins called the MICALs that are required for Sema/Plexin signal transduction. Now, recent work in my
lab has revealed that the MICALs employ a previously unknown Redox signaling system to control the actin
cytoskeleton. Namely, we have found that Mical is a novel F-actin disassembly factor – and our results reveal
that Sema/Plexin-mediated reorganizations of the actin cytoskeleton can be precisely achieved in space and time
through activation of Mical. We have also found that the MICALs belong to a class of oxidoreductase (Redox)
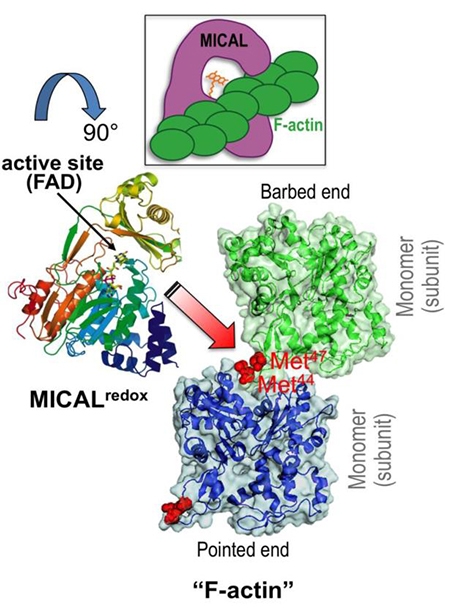
enzymes and that Mical employs its Redox enzymatic activity to alter the properties of F-actin. Our work has
gone on to identify that Mical uses F-actin as a direct substrate and post-translationally oxidizes conserved amino
acids on actin, simultaneously dismantling F-actin and decreasing polymerization. Moreover, we find that this
Sema/Plex/Mical-mediated Redox regulation of actin is reversible (by a protein called SelR/MsrB) – and that this
specific reversible Redox actin regulatory system directs multiple different biological processes in neuronal and
non-neuronal tissues. Thus, MICALs and SelR/MsrBs form a reversible Redox cellular signaling system controlling
actin filaments – the building blocks of neuronal form and function.