You are here
Fibronectin is a Proximal Mediator of APOE-ε4-induced Blood-Brain Barrier Dysfunction in Alzheimer’s Disease
Speakers
Abstract
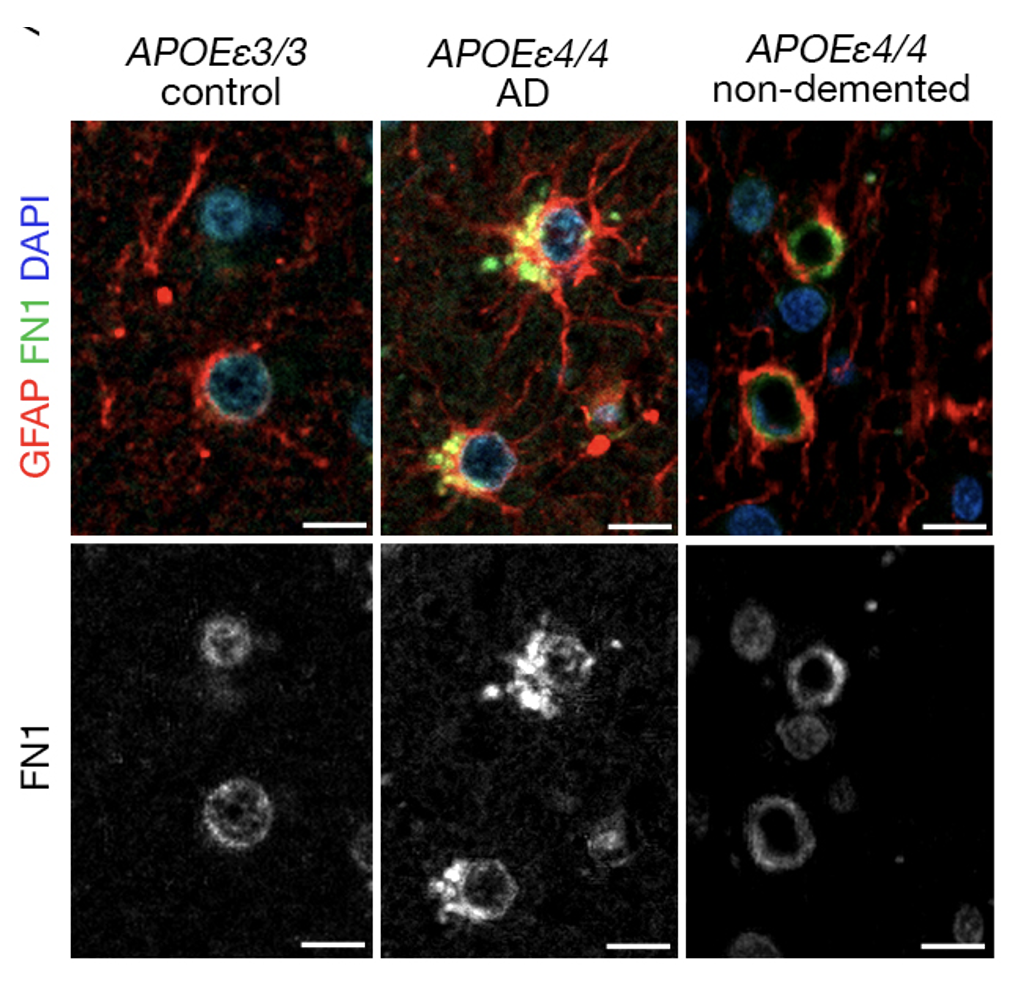
The APOE-ε4 allele is the strongest genetic risk factor for late-onset Alzheimer’s disease, in part by driving early blood-brain barrier (BBB) breakdown, perivascular inflammation, and pathological extracellular matrix (ECM) remodeling. Excessive fibronectin deposition at the BBB basement membrane impairs endothelial–astrocyte crosstalk, disrupts tight junctions, and amplifies neuroinflammation, thereby accelerating cognitive decline in APOE-ε4 carriers. Through whole-genome sequencing of >10,000 APOE-ε4 homozygotes, we identified two rare FN1 variants that segregate exclusively with cognitively resilient individuals. Functional studies in iPSC-derived astrocyte– endothelial co-cultures and orthologous zebrafish models demonstrate that one genetic variant reduces pathogenic fibronectin fibrillogenesis at the BBB, restores tight-junction integrity, normalizes VEGFA signaling, and attenuates immune cell recruitment. Multi-omic profiling further reveals that FN1 variant remodels the vascular lipidome and transcriptome toward a homeostatic, anti-inflammatory state, providing mechanistic insight into variant-driven resilience. By pinpointing fibronectin as a proximal effector of APOE-ε4–induced BBB pathology, these findings establish FN1 as a tractable target for therapeutic intervention. In this presentation, I will discuss our genetic and mechanistic dissection of fibronectin’s role in BBB health, outline how naturally protective FN1 variants confer resilience in APOE-ε4 carriers, and highlight the therapeutic potential of targeting fibronectin–integrin interactions to preserve vascular integrity and prevent cognitive decline in Alzheimer’s disease.