You are here
Arginine Methylation: A novel post translational modification that impacts Huntington function in Huntington’s disease
Speakers
Abstract
Huntington’s disease (HD) is a fatal neurodegenerative disorder characterized by the loss of striatal and cortical neurons. HD is caused by an abnormal polyglutamine (polyQ) expansion in huntingtin protein (HTT). Arginine methylation is a post-translational modification (PTM) catalyzed by protein arginine methyltransferases (PRMTs) with a key role in the pathogenesis of polyglutamine diseases. However, its role in HD remains to be elucidated.
We analyzed arginine methylation of HTT by mass spectrometry of mouse brain extracts and in vitro methylation assays. The interaction of wild-type or polyQ-HTT with PRMTs was investigated through immunoprecipitation and proximity ligation assay in immortalized striatal cells. Finally, we undertook a loss- of-function and gain-of-function approach both in vitro and in vivo to assess the role of PRMTs and arginine methylation in HD. We found that HTT is methylated at specific arginine residues in the brain of normal mice. HTT colocalizes and forms a complex with specific members of the PRMT family. Importantly, the polyglutamine expansion in HTT reduces the interaction with the PRMTs. Loss of PRMT function in striatal cells expressing polyQ-HTT exacerbates toxicity, and the expression of a methylation-defective mutant of HTT decreases the survival of primary cortical neurons. Conversely, gain of PRMT function attenuates striatal cell death and suppresses polyQ-HTT-mediated lethality in HD flies. These results suggest that arginine methylation plays a key role in HD pathogenesis. Particularly, PRMT-mediated arginine methylation of HTT is protective in HD.
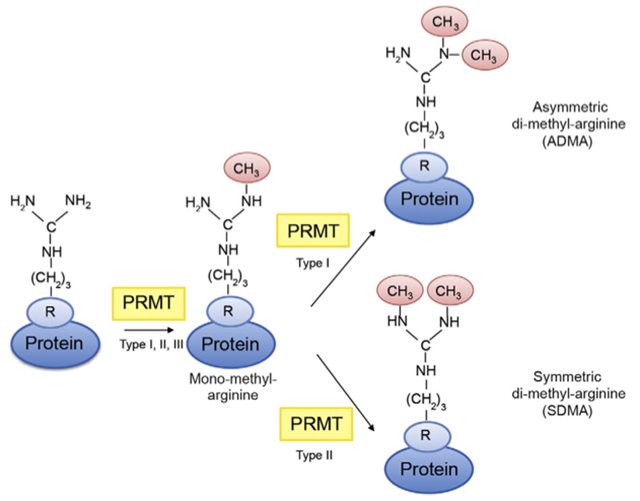
Arginine methylation by PRMTs catalyze the transfer of a methyl group to the side chain of arginine residues, thereby generating mono-methylarginine. Addition of another methyl group to the same guanidine group generates asymmetric di-methyl-arginine, a reaction catalyzed by type I PRMTs, whereas addition of the methyl group to the other guanidine group generates symmetric di-methyl-arginine, which is catalyzed by type II PRMTs.

