You are here
Using tDCS in Speech-Based Stroke Rehabilitation
CLINICAL TRIAL:
In-Progress / Currently Recruiting ParticipantsFlyer:

Qualifying participants who have had a stroke and have apraxia of speech will receive up to 24 speech therapy sessions over a 4 to 6 month period, at no charge.
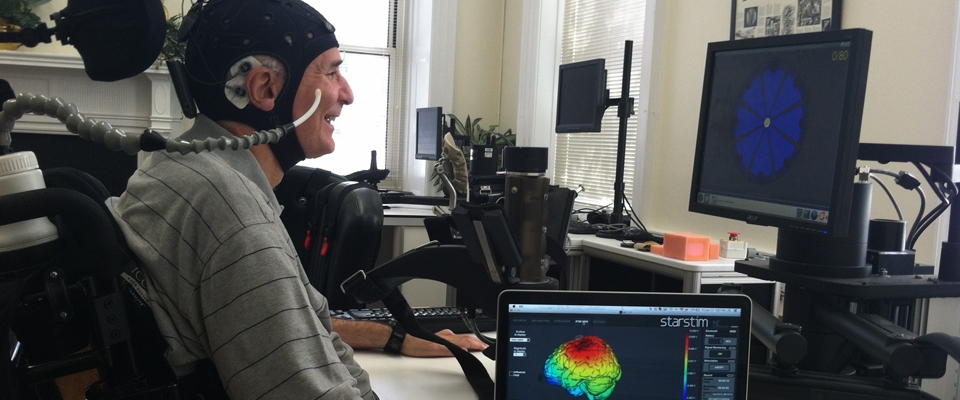
Transcranial direct current stimulation (tDCS), a form of non-invasive brain stimulation, will also be used during a portion of speech therapy. tDCS is considered a non-risk device by the FDA, and is safe to use. Low doses of electrical current will be administered through the scalp over areas that may assist with speech recovery.
Eligibility
Inclusion Criteria:
For patients with stroke:
- Have experienced a stroke and have apraxia of speech